To Calculate the Molarity of a Solution
This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration (or recalculating grams per ml to moles). Yous can also calculate the mass of a substance needed to reach a desired molarity. This commodity volition provide yous with the molarity definition and the molarity formula.
To understand the topic as a whole, you will want to acquire the mole definition, read a paragraph about the molarity units, as well equally read a comparison of two misleading concepts: molarity formula vs molality formula. What is more, nosotros prepared for you some interesting examples of tooth solutions and a brusk stride-by-step tutorial of how to calculate molarity of a concentrated solution.
At the end, you tin learn the titration definition and find how to find the molar concentration using the titration process, which may be helpful when carrying out titrations!
Molar concentration – an introduction
When you wait around, even if yous're sitting at home, you volition notice many dissimilar objects. The majority of these materials are not pure. They are, in fact, mixtures.
Mixtures consist of a drove of different compounds. Occasionally, the number of elements may be quite loftier, or sometimes quite depression, but as long equally there is more one element in an object, it is a mixture. Orange juice in your glass, a loving cup of tea, detergents in the bathroom or milk – all these substances are mixtures.
Mixtures are non express to but liquids though, solids and gases can both be mixtures; even biological organisms are very complex mixtures of molecules, gases, and ions dissolved in h2o.
In chemistry, there are 2 types of mixtures:
-
Homogeneous mixtures – Components are uniformly distributed throughout the mixture, and at that place is but one phase of matter observed. They are also known as solutions and may occur in the solid, liquid or gaseous state. It is not possible to merely separate the mixture components, just no chemical change has occurred to any of the components. Examples: sugar h2o, dishwashing detergent, steel, windshield washer fluid, air.
-
Heterogeneous mixtures – Components of the mixture are not uniformly distributed and may have regions with different properties. Unlike samples of the mixture are not identical. At least two phases are e'er present in the mixture, and it's usually possible to physically separate them. A few examples of such substances: blood, concrete, ice cubes in cola, pizza, the Pacific Sea.
Concentration is one of the nigh well known and most important parameters for anybody who works with whatever chemical substances or reactions. It measures how much of a substance is dissolved in a given volume of solution.
Chemists employ many dissimilar units for describing concentration. Nonetheless, the term molarity, likewise known as molar concentration, is the most common way of expressing the concentration. When the reactants (compounds) are expressed in mole units, it allows them to be written with integers in chemic reactions. This helps to easily work with their amounts. Starting time, allow's accept a closer expect at what is the mole, so nosotros can move on later to find what is molarity.
Mole definition
The mole is the SI unit of measurement for the amount of substance. The current definition was adopted in 1971 and is based on carbon-12. It says:
"The mole is the amount of substance of a system which contains as many uncomplicated entities every bit at that place are atoms in 0.012 kilograms of carbon-12; its symbol is "mol". When the mole is used, the elementary entities must exist specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles."
It follows that the molar mass of carbon-12 is exactly 12 grams per mole, M(¹²C) = 12 g/mol. The word "substance" in the definition should specify (be replaced with the proper noun of) the substance concerned in a particular application, e.grand., the amount of chloride (HCl) or the amount of carbon dioxide (CO₂). It is crucial to e'er requite a precise specification of the entity involved (every bit noted in the second role of the mole definition). This should be done by providing the empirical chemical formula of the compound involved.
According to the newest conventions (effective as of the twentyth May 2019), the mole definition is that a mole is the amount of a chemical substance that contains exactly 6.02214076 × 1023 particles, such as atoms, molecules, ions etc. That number is known as Avogadro's constant. Its symbol is NA or L. Using the Avogadro number provides a convenient way of considering the weight of substance and the theoretical yield of chemical reactions. Moles permit y'all to directly read weight from the periodic table (eastward.thousand., one mole of N₂ is 28 yard or 1 mole of NaCl is 58.5 g).
We can link the number of entities 10 in a specified sample – N(10), to the moles of X in the same sample – n(X), with the relation: north(X) = N(X)/NA. Northward(X) is dimensionless, and northward(X) has the SI unit of measurement mole.
What is molarity?
So yous are not confused with similar chemical terms, proceed in mind that molarity ways exactly the same as molar concentration (Chiliad). Molarity expresses the concentration of a solution. It is defined every bit the number of moles of a substance or solute, dissolved per liter of solution (not per liter of solvent!).
concentration = number of moles / volume
Molarity formula
The post-obit equation allows you to discover the molarity of a solution:
molarity = concentration / molar mass
The concentration denotes the mass concentration of the solution, expressed in units of density (usually g/fifty or 1000/ml).
Tooth mass is the mass of one mole of the solute. Information technology is expressed in grams per mole. It is a constant holding of each substance – for example, the molar mass of h2o is approximately equal to 18 k/mol.
Our estimator tin likewise observe the mass of substance you lot need to add to your solution to obtain a desired tooth concentration, according to the formula:
mass / volume = concentration = molarity * molar mass
where mass is the mass of solute (substance) in grams, and volume is the total volume of solution in liters.
Molarity has many applications. One of them is the calculating the solution dilution.
Molarity units
The units of molar concentration are moles per cubic decimeter. They are noted as mol/dm³ likewise as K (pronounced "molar"). The tooth concentration of solute is sometimes abbreviated by putting square brackets around the chemical formula of the solute, e.g., the concentration of hydroxide anions tin be written as [OH⁻]. In many older books or articles, you can find different units of molar solutions – moles per liter (mol/50). Remember that one cubic decimeter equals to ane liter, so these two notations express the same numeric values.
Formerly, chemists used to give concentrations as the weight of solute/volume. Present, since mole has become the nearly common way of quoting the quantity of a chemical substance, molarity is unremarkably used instead.
Note that molarity might be quite often confused with the term molality. Molality is usually written with lower example k, while molarity (what was mentioned in a higher place) with an uppercase G. Nosotros explain the difference between these two in a paragraph below.
Molarity besides plays a significant function in calculating the ionic strength of a solution.
How to calculate molarity
- Choose your substance. Let'southward assume that it is the muriatic acid (HCl).
- Find the molar mass of your substance. For the hydrochloric acid, it is equal to 36.46 grand/mol.
- Decide on the mass concentration of your substance – you can either input it directly or fill in the boxes for substance mass and solution book. Let'due south presume that you lot have five yard of HCl in a i.two liter solution.
- Convert the expressions in a higher place to obtain a molarity formula. Equally
mass / volume = molarity * tooth mass, thenmass / (volume * molar mass) = molarity. - Substitute the known values to calculate the molarity:
molarity = 5 / (1.2 * 36.46) = 0.114 mol/l = 0.114 Thou. - You tin also utilise this molarity computer to notice the mass concentration or molar mass. Simply type in the remaining values and watch it practise all the work for you.
Molarity vs molality
Permit's consider the differences betwixt these two similarly named chemic concepts: molarity and molality. We promise that after reading this paragraph, you will have no doubts regarding this topic.
Both terms are used to express the concentration of a solution, but there is a significant difference between them. While molarity describes the corporeality of substance per unit volume of solution, molality defines the concentration equally the amount of substance per unit mass of the solvent. In other words, molality is the number of moles of solute (dissolved material) per kilogram of solvent (where the solute is dissolved in).
It is possible to recalculate from molarity to molality and vice versa. To make this shift, utilise the formula beneath:
molarity = (molality * mass_density_of_the_solution) / (1 + (molality * molar_mass_of_the_solute))
In this molarity vs molality tabular array, you tin detect all chief differences betwixt these two terms:
| Molarity | Molality | |
|---|---|---|
| Definition | Amount of substance (in moles) divided past the volume (in litres) of the solution | Amount of substance (in moles) divided by the mass (in kg) of the solvent |
| Symbol | Grand | m or b |
| Unit | mol/L | mol/kg |
| [Temperature](calc:206) and pressure | Dependent | Independent |
| Usage | More than popular, practical to utilize in the lab, faster and easier | Accurate but rarely used |
Tooth solution – life examples
Equally you already know, mixtures and solutions e'er environs the states, and they are a permanent role of the environment. In the table below, you lot can find the list of orders of magnitude for molar concentration, with examples taken from the natural environment.
| Molarity | SI prefix | Value | Detail |
|---|---|---|---|
| 10⁻¹⁵ | fM | 2 fM | Bacteria in surface seawater (i×10⁹/L) |
| 10⁻¹⁴ | – | 50–100 fM | [Gold](calc:531) in seawater |
| 10⁻¹² | pM | 7.51–ix.80 pM | [Normal](calc:472) range for erythrocytes in blood in an adult male |
| ten⁻⁷ | – | 101 nM | Hydronium and hydroxide ions in pure water at 25 °C |
| 10⁻⁴ | – | 180–480 µM | Normal range for [uric acid](calc:755) in [blood](calc:760) |
| x⁻³ | mM | 7.8 mM | Upper leap for healthy [blood glucose](calc:737) 2 hours after eating |
| ten⁻² | cM | 44.6 mM | Pure [ideal gas](calc:435) at 0 °C and 101.325 kPa |
| 10⁻¹ | dM | 140 mM | [Sodium ions in blood plasma](calc:822) |
| 10² | hM | 118.eight Grand | Pure osmium at xx °C (22.587 g/cm³) |
| 10⁴ | hM | 24 kM | [Helium](calc:975) in the solar cadre (150 g/cm³ * 65%) |
Determining the molar concentration by titration
Titration is a technique with which you can find the concentration of an unknown solution, based on its chemical reaction with a solution with a known concentration. This process is based on calculation the titrant (with a known concentration & book) to a known quantity of the unknown solution (the analyte) till the reaction is complete. Y'all can then make up one's mind the concentration of the analyte past measuring the volume of titrant used.
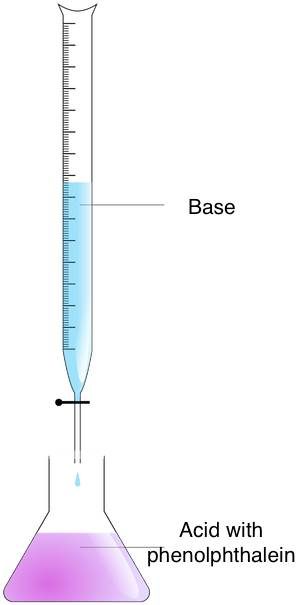
Follow these steps to discover the molarity of an unknown solution with the titration method:
- Prepare the concentrations – Put the analyte in an Erlenmeyer flask and the titrant in a burette.
- Mix the concentrations – Add the titrant to the analyte until the endpoint is reached. You can observe this moment by observing the color alter. Apply the acid-base of operations indicator for this purpose. If you accept used phenolphthalein, you will discover a color change from pink to colorless.
- Calculate the molarity – Utilize the titration formula. If the titrant to analyte ratio is 1:1, apply the equation:
acid_molarity * acid_volume = molarity_of_base * volume_of_base.
For ratios other than 1:one, y'all demand to change the formula.
Example: 35 ml of i.25 M HCl acrid is needed to titrate a 25 ml solution of NaOH. In that case, you can use the i:1 formula because one mole of HCl reacts with 1 mole of NaOH. Then, multiply the molarity of the acrid by the volume of the acid – 1.25 * 35 = 43.75 and the effect, past the volume of the base of operations. The molarity of the base equals 43.75 / 25 = 1.75 Thou.
You tin can also determine the molar concentration of a solution by using the Beer–Lambert–Bouguer law.
Make certain you bank check out our alligation figurer if yous are interested in determining how to obtain different concentrations of a solution.
FAQ
How practice I summate pH from molarity?
- Summate the concentration of the acid/element of group i component of your solution.
- Calculate the concentration of H+ or OH- in your solution if your solution is acidic or alkaline metal, respectively.
- Work out -log[H+] for acidic solutions. The result is pH.
- For alkaline metal solutions, observe -log[OH-] and decrease information technology from 14.
How do yous make a molar solution?
- Find the molecular weight of the substance y'all'd like to make a molar solution of in g/mol.
- Multiply the molecular weight of the substance by the number of moles you wish to accept, which in this case is one.
- Weigh out the number of grams you lot calculated in pace 2 of your substance and identify it in a container.
- Measure out 1 liter of your chosen solvent and add information technology to the same container. You lot now take a molar solution.
What is molar volume?
Tooth volume is the volume that one mole of a substance takes upwards at a detail temperature and pressure. It is found by dividing the molar mass by the substance's density at that temperature and pressure level.
How practice I find moles from molarity?
- Find the molarity and volume of your solution.
- Make certain that the units for the volume are the same as for the volume part of the molarity (e.g., mL and mol/mL).
- Multiply the volume by the molarity. This is the number of moles present.
Is molarity the aforementioned equally concentration?
Molarity is not the same every bit concentration, although they are very like. Concentration is a mensurate of how many moles of a substance are dissolved in an corporeality of liquid, and tin take any book units. Molarity is a type of concentration, specifically moles per liter of solution.
How exercise y'all brand a molar solution?
- Detect the molecular weight of the substance y'all'd like to make a molar solution of in thousand/mol.
- Multiply the molecular weight of the substance by the number of moles you lot wish to have, which in this case is 1.
- Weigh out the number of grams you calculated in stride two of your substance and place it in a container.
- Measure out out 1 liter of your called solvent and add together it to the same container. You now have a molar solution.
What is the molarity of water?
H2o has a molarity of 55.5 M. 1 liter of water weighs 1000 g, and, every bit molarity is the number of moles per liter; finding the molarity of water is the same as finding the number of moles of water in 1000 g. We therefore separate the weight by the molar mass to get moles, one thousand / 18.02 = 55.v M.
Why practise we use molarity?
Molarity is a helpful measure to use when discussing concentration. As concentration has a big range of sizes of units, from nanogram per milliliter to ton per gallon, it is easier to accept a known metric for quick comparing of concentrations without having to deal with conversions. This is molarity (K), which is moles per liter.
Source: https://www.omnicalculator.com/chemistry/molarity
0 Response to "To Calculate the Molarity of a Solution"
Enregistrer un commentaire